The EFSA published on May 2022 an article related to the project “Microbiota analysis for risk assessment: evaluation of hazardous dietary substances and its potential role on the gut microbiome variability and dysbiosis” in a special issue dedicated to the European Food Risk assessment (EU-FOR A) program.
Considering the development of fields related to probiotics, microbiome-targeted interventions, there is a need to clearly specify criteria and provide details about ways and approaches of achieving those criteria with the intention that manufacturers can launch new products and benefit from a transparent way of communicating product quality to end users.
The proposed project of “Microbiota analysis for risk assessment improval: Evaluation of hazardous dietary substances and its potential role on the gut microbiome variability and dysbiosis” was developed within Faculty of Pharmacy, University of Granada (UGR), and “Jose Mataix Verdu” Institute of Nutrition and Food Technology (INYTA – UGR) team projects that carry out microbiota analysis with different health purposes since 2003. The current interest of gut microbiota determinations for complementing risk assessment of metabolite traces of toxicants and xenobiotic substances in food is being of high relevance. Within this initiative, multidisciplinary consortium submitted the following EFSA Partnering Grant proposal (2019–2021) that has been successfully evaluated and awarded: “KNOWLEDGE PLATFORM FOR ASSESSING THE RISK OF BISPHENOLS ON GUT MICROBIOTA AND ITS ROLE IN OBESOGENIC PHENOTYPE: LOOKING FOR BIOMARKERS” Acronym: OBEMIRISK. The program was supervised by Dr. “Margarita Aguilera-Gomez”, Associate Professor at UGR. The program consisted of three different independent modules based on on-going research project work and previous research interests:
- Objective/Module 1. To focus on obtaining the upmost information about human microbiota variability and dysbiosis associated and/or putatively caused by diet hazardous substances exposure and consumption, and future perspectives of probiotics and next-generation probiotics.
A systematic review, a meta-analysis and an extensive literature search methodology was taught and applied to obtain relevant documents for the holistic analysis of human microbiota and its role in risk assessment and to build Guidelines documents compiling Regulatory data and scientific evidence affecting microbiota and probiotics risk assessment and food safety aspects. The regulation of marketed probiotics differs among countries. They can be launched a s nutraceuticals or life biotherapeutic agents, medical foods, biological products or food supplements according to the country. In addition, the basic level of classification is not globally harmonized.
- Objective/Module 2. To learn main available methods and omics technologies for gut microbiota analysis (composition/activity patterns) while exposed to different level of diet hazardous substances (e.g. bisphenol A and analogues).
- Objective/Module 3. To learn main methods for chemical determination of bisphenols and analogues in food samples and human sample specimens (saliva, urine, faeces) and elaboration of common questionnaires and surveys for food exposure estimation of the presence of BPA and analogues (design, improvement, validation and its implementation).
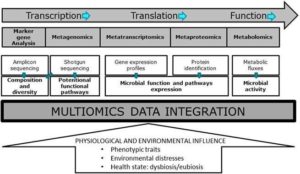 Integrating multi-omics datasets is an innovative assignment , due to the increased complexity and diversity of the collected data. This integration is increasingly reliant on efficient bioinformatics tools and advanced statistical methods. Therefore, multi-omics data integration still poses challenges, but integration of multiple metaomics datasets lays out a promising approach to comprehensively characterising the composition, functional, and metabolic activity of microbiomes. This is of particular importance for microbiome research to be translated into clinical applications, together with an increased demand for larger prospective cohort studies to validate findings and determine biomarker reproducibility before they can find applications for further improvement of human health management (see the figure below, extracted from the EFSA Journal).
Integrating multi-omics datasets is an innovative assignment , due to the increased complexity and diversity of the collected data. This integration is increasingly reliant on efficient bioinformatics tools and advanced statistical methods. Therefore, multi-omics data integration still poses challenges, but integration of multiple metaomics datasets lays out a promising approach to comprehensively characterising the composition, functional, and metabolic activity of microbiomes. This is of particular importance for microbiome research to be translated into clinical applications, together with an increased demand for larger prospective cohort studies to validate findings and determine biomarker reproducibility before they can find applications for further improvement of human health management (see the figure below, extracted from the EFSA Journal).
The research program included collection and analysis of faecal samples from children (n=109) population, aged between 3 and13 years old, clustered according to different level of bisphenols and analogues measurement in order to identify microbiota composition, and dysbiosis phenotypes patterns. Enterotypes of gut microbiota have also been explored. Microbiome data collection, collation, comparison and integration of data contribute to a deeper knowledge on dysbiosis phenotype-xenobiotic/toxic compound. The results of this research program will be published in open access publications.
To know more about this project, you can consult this link.
Orchidali can help on launching probiotic strain for the French and European market.

Microbiota analysis for risk assessment
The EFSA published on May 2022 an article related to the project “Microbiota analysis for risk assessment: evaluation of hazardous dietary substances and its potential role on the gut microbiome variability and dysbiosis” in a special issue dedicated to the European Food Risk assessment (EU-FOR A) program.
Considering the development of fields related to probiotics, microbiome-targeted interventions, there is a need to clearly specify criteria and provide details about ways and approaches of achieving those criteria with the intention that manufacturers can launch new products and benefit from a transparent way of communicating product quality to end users.
The proposed project of “Microbiota analysis for risk assessment improval: Evaluation of hazardous dietary substances and its potential role on the gut microbiome variability and dysbiosis” was developed within Faculty of Pharmacy, University of Granada (UGR), and “Jose Mataix Verdu” Institute of Nutrition and Food Technology (INYTA – UGR) team projects that carry out microbiota analysis with different health purposes since 2003. The current interest of gut microbiota determinations for complementing risk assessment of metabolite traces of toxicants and xenobiotic substances in food is being of high relevance. Within this initiative, multidisciplinary consortium submitted the following EFSA Partnering Grant proposal (2019–2021) that has been successfully evaluated and awarded: “KNOWLEDGE PLATFORM FOR ASSESSING THE RISK OF BISPHENOLS ON GUT MICROBIOTA AND ITS ROLE IN OBESOGENIC PHENOTYPE: LOOKING FOR BIOMARKERS” Acronym: OBEMIRISK. The program was supervised by Dr. “Margarita Aguilera-Gomez”, Associate Professor at UGR. The program consisted of three different independent modules based on on-going research project work and previous research interests:
A systematic review, a meta-analysis and an extensive literature search methodology was taught and applied to obtain relevant documents for the holistic analysis of human microbiota and its role in risk assessment and to build Guidelines documents compiling Regulatory data and scientific evidence affecting microbiota and probiotics risk assessment and food safety aspects. The regulation of marketed probiotics differs among countries. They can be launched a s nutraceuticals or life biotherapeutic agents, medical foods, biological products or food supplements according to the country. In addition, the basic level of classification is not globally harmonized.
The research program included collection and analysis of faecal samples from children (n=109) population, aged between 3 and13 years old, clustered according to different level of bisphenols and analogues measurement in order to identify microbiota composition, and dysbiosis phenotypes patterns. Enterotypes of gut microbiota have also been explored. Microbiome data collection, collation, comparison and integration of data contribute to a deeper knowledge on dysbiosis phenotype-xenobiotic/toxic compound. The results of this research program will be published in open access publications.
To know more about this project, you can consult this link.
Orchidali can help on launching probiotic strain for the French and European market.
Tags: